For years, researchers considered Alzheimer’s disease (AD) an amyloid beta and neuron-related condition. But having trained as a pathologist, Thomas Montine, who is now a neuropathology researcher at Stanford University, didn’t think that was the whole story.
“It was the pathologist in me that kept asking, ‘But where did the brain tissue go?’” he said. “When muscle atrophies, you can tell. You can see it histologically,” he said. “Brain? It’s just gone.”
In fact, the neurodegenerative disease reminded him of something more like tuberculosis.
“Tuberculosis used to be called consumption because the lung was consumed. It was the same idea. The tissue was gone,” he said. “In the early 90s, it started us down the road of immune cell-mediated consumption…and in the brain, that’s microglia.”
Now decades later, neuroscientists recognize the important role microglia play in keeping the brain healthy as well as how the cells contribute to neurodegenerative disorders like AD.1 However, most microglia studies in humans have profiled just a few brain sections, and rodent microglia do not fully recapitulate human microglia biology.

Sean Bendall (left) and Thomas Montine (right) used multiplexed ion beam imaging to create a spatial proteomic atlas of postmortem human brains from both cognitively healthy people and people with Alzheimer’s disease.
Sean Bendall and Thomas Montine
To fill those research gaps, Montine, his Stanford University colleague Sean Bendall, and their teams, used high-resolution imaging to create a spatial proteomic atlas of microglia in the postmortem brains of both cognitively healthy people and people with AD.2 They discovered that microglia exist on a continuum of functional states that vary across the brain anatomy and that some microglia in AD brains appeared immunologically exhausted, presenting new therapeutic opportunities. The team published their findings in Nature Immunology.
“It’s really a landmark study,” said David Gate, a neuroimmunologist at Northwestern University who was not associated with the research. “One of the exciting things about this study is just how high throughput it is, and the resolution, of course, is very high.”
Self-described as a “blood and immune guy,” Bendall and his team develop single-cell imaging technologies to better understand these systems. “Right around the time Tom showed up at Stanford, we had just come up with a new mass spec-based tissue imaging technology that I think Tom immediately recognized could have some play with CNS tissue,” Bendall said. That tool, called multiplexed ion beam imaging (MIBI), works similarly to confocal microscopy, but instead of using a laser and fluorophores, it uses a nanometer-scale ion beam and elemental isotopes, respectively.3
Archival brain tissues—which are pretty much the only human samples available for neurodegenerative disease research—are technically difficult to work with, Bendall said. Nucleic acids, particularly RNA, will typically have already degraded by the time researchers want to image the samples. Cholesterol and other aromatic compounds build up in these tissues with age, which causes autofluorescence, making light-based imaging challenging. Furthermore, microglia are rare in the overall population of the brain and quite small, so researchers need to use high-resolution techniques to image them. With MIBI’s high resolving power and its readout being the presence or absence of an atom, the researchers can both capture the microglia and not worry about any background signal or autofluorescence.
Using MIBI, the team examined tissue from multiple regions of the postmortem brains of 10 cognitively normal people and 13 AD age-matched ones. Studying cognitively normal brains, they found that microglia exist on a spectrum of immune activation that varied based on where in the brain the cells were spatially located. “Each part of the brain likely has kind of sub-specialized flavors of microglia, and this is consistent between individuals,” said Bendall.
To gain an additional layer of microglial functional information, reviewers had asked the team to do spatial transcriptomics of these samples as well, which, Bendall said, “Tom and I knew from history and from working with these types of samples [that it] was going to be a no-go from the start.”
Fortunately, study coauthor and neuro-epigenomics researcher Ryan Corces, who is at the Gladstone Institutes, had recently published epigenomic profiles of cells in the brain.4 When the team looked specifically at the microglia epigenomic data, they saw the same functional continuum.
In the AD brains, while the researchers were not surprised to see microglia expressing markers of disease-associated microglia (DAMs) around amyloid plaques and tangles, they were shocked that these cells made up only about one percent of the total microglia in the AD brain.

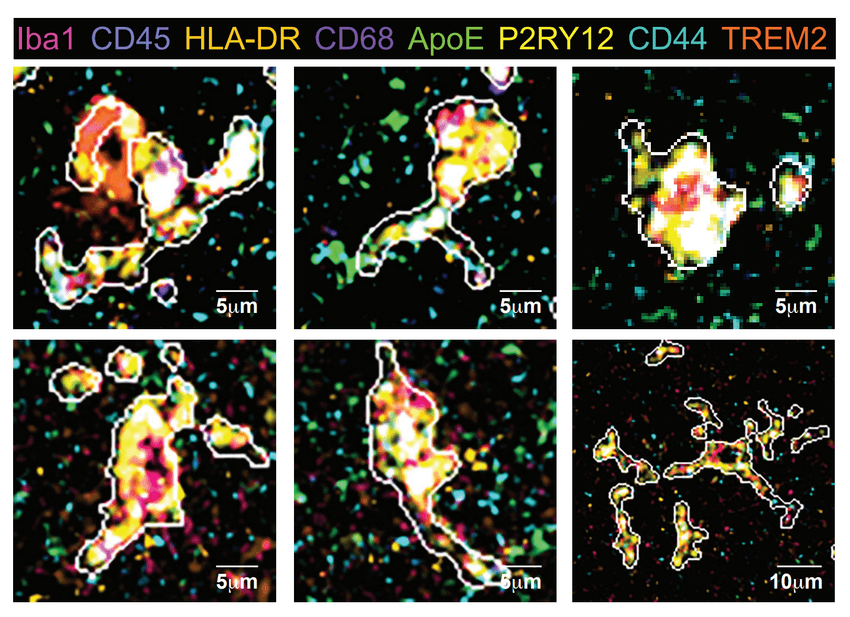
Microglial states in an AD human hippocampus are shown in a multiplexed ion beam imaging example. The eight channel pseudo-color indicates the expression of each protein. The predicted cell boarder is shown with a white line.
Kausalia Vijayaragavan
“There was actually a bigger swath of microglia, more subtly, there, that were also shifting in their behavior,” said Bendall. These AD microglia had decreased expression of proteins involved in T cell activation and antigen presentation as well as increased expression of immune suppression markers. In fact, these microglia looked like cells Bendall had seen before—but not ones in the brain.
“The closest thing I’ve seen to this in my broader studies is looking at T cells in a tumor microenvironment,” said Bendall. “Classically, we would think these cells look inflamed; they look activated, but we actually know now—by virtue of the fact that the tumor is still there—that those cells are actually, we call it, exhausted.”
The team reasoned that evolutionarily, it would be important for this microglial exhaustion to occur to protect the body from a constantly activated immune system.
“Think about all of the things that we hear about that help prevent Alzheimer’s disease and other neurodegeneration, which is like: lower your stress, lower your inflammation, lower incidence of chronic infection and disease. So basically, keep inflammation throughout life down,” said Bendall. “Chronic inflammation leads to exhaustion. We know this in infection. We know this in tuberculosis. We know this in cancer.”
Oleg Butovsky, a neuroimmunologist at Harvard University who was not involved in the research, thought this finding was fascinating too. “I love the conclusion [that], based on the finding, they suggested [there is a] functional or exhausted immune profile,” said Butovsky. “We need to find why in Alzheimer’s these microglia get to an exhausted state…Can we find a way to restore their normal phenotype?”
Now that the field has this spatial proteomic atlas of microglia, both Butovsky and Gate want to know how AD genetic risk factors affect microglia and their function in the brain. Montine and Bendall have already begun digging into these questions. In particular, they’re excited to look at the microglia from so called “resilient” people with AD: those who were cognitively normal during life, but upon death, show clear AD brain pathology.
“If you were to show [the brain] to a pathologist, the pathologist would say, ‘Oh, this person must have had dementia,’” explained Montine. “They’re cognitively appropriate for age, yet they have a head full of plaques and tangles, so how do they possibly pull that off? I mean, there’s a major therapeutic clue there.” They’re eager to see what the spatial proteomes of the microglia in these patients say about both AD and aging more generally.
“We’ve got way too much invested in building this to stop,” said Montine. Bendall added, “It puts a couple points on the board for the thesis that Tom brought up at the beginning, which is this idea that microglia could be playing more of a role [in AD] and that we’ve identified some of the behavior that seems to be going wrong.”
Source link